
FOCAS Trial: Focal Cerebral Arteriopathy Steroids (FOCAS)
STUDY OVERVIEW:
Overall goal:
To test the hypothesis that among children with suspected focal cerebral arteriopathy (FCA), early corticosteroid treatment to prevent FCA progression (Study Arm A) leads to overall better outcomes than selective, delayed treatment in only the subset that progresses (Study Arm B).
Background:
FCA is an acute, monophasic, presumed inflammatory disease that causes unilateral stenosis of the intracranial anterior circulation. Although rare (1 to 3 cases seen per year at a typical academic children’s hospital), it is one of the most common causes of arterial ischemic stroke in a previously healthy child (median age 7 years; occurs in infants to adolescents). Pediatric stroke investigators identified an FCA treatment trial as the #1 priority of the field (Steinlin M, Dev Med Child Neurol 2017) because of its aggressive natural history: it often progresses dramatically over days to weeks with recurrent or expanding infarcts. A 2017 European retrospective cohort study suggested that corticosteroid treatment may improve outcomes for FCA (Steinlin M, Stroke 2017).
 |  |  |
|
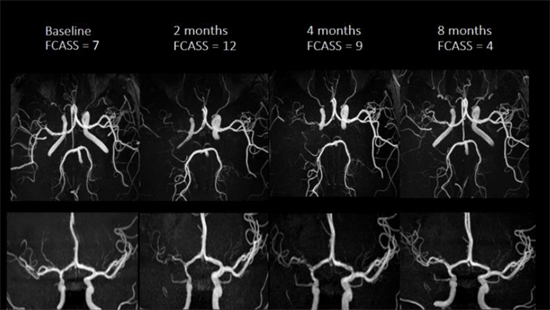
 | Serial MRAs from a typical case of FCA in the Vascular effects of Infection in Pediatric Stroke (VIPS) Study. The arteriopathy progressed initially, with a rising FCA Severity Score (FCASS) from baseline to 2 months, then improves. (Ref: Fullerton HJ, Wintermark M, et al, Stroke 2018. PMID: 30355212).
|  |
 |
Europeans have begun the PASTA (Paediatric Arteriopathy STeroid Aspirin) trial (PI Steinlin): a “gold-standard” RCT to test the efficacy of corticosteroids for FCA. A survey of VIPS investigators in 2018 revealed discomfort with randomization to “no steroids” as the majority now treat FCA with corticosteroids. However, despite our attitudes favoring their use, corticosteroids were given to only 36% of 55 children with suspected FCA in the VIPS II cohort and (when given) were started a median of 3 days post-stroke (IQR 1.5, 6). This incongruity reflects diagnostic uncertainty at stroke baseline: the characteristic arteriopathy evolution is needed for definitive diagnosis of FCA, and ≈1 in 5 children with suspected FCA at baseline have an alternate diagnosis. Hence, the pressing clinical question is:
Should we treat all children with suspected FCA immediately or wait and treat only the subset that demonstrate disease progression?
Early treatment has the potential advantage of preventing FCA progression, but the disadvantage of overtreatment of those with alternate diagnoses. With a comparative effectiveness approach, we can compare these two treatment approaches. We also need safety data to guide this decision, particularly data on risk of severe infection or symptomatic intracerebral hemorrhage (sICH) related to steroid-induced hypertension.
Study Design: Comparative Effectiveness Trial
Efficacy vs. effectiveness: Randomized controlled trials (RCTs) measure efficacy of an intervention in a highly controlled, “ideal” setting. Comparative effectiveness studies measure effectiveness in a “real world” setting, where the intervention is being implemented.
Over a 5.5-year enrollment period, the Focal Cerebral Arteriopathy Steroid (FOCAS) Trial will enroll 80 children with suspected FCA and centrally randomize them 1:1 to either Study Arm A or B.
- Study Arm A: Treat all children with suspected FCA with corticosteroids as soon as the diagnosis is made.
- Study Arm B: Treat only the subset of children that develop evidence of FCA disease progression.
Inclusion Criteria:
- Age 1 year through 18 years at stroke/TIA ictus (ineligible as of 19th birthday).
- Acute arterial ischemic stroke (AIS) or transient ischemic attack (TIA) in prior 4 days (96 hours).
- AIS definition: neurological deficit with acute onset (including seizures) and acute infarct(s) corresponding to arterial territory(ies) on brain imaging.
- TIA definition: neurological deficit with acute onset (not including seizures) consistent with ischemia of an arterial territory(ies) but without acute infarction on brain imaging.
- Imaging inclusion criteria:
- Baseline imaging findings consistent with FCA:
- unilateral focal irregularity, banding, stenosis, wall thickening/enhancement, or occlusion of the distal internal carotid artery (ICA) and/or its proximal branches (A1, M1, posterior communicating artery, proximal PCA), OR
- unilateral infarction in the territory of the lenticulostriate arteries with normal MRA.
- Ability to return at 1-month (±7 days) post-stroke for an MRA (non-contrast) on a scanner of the same magnet strength as baseline MRI/MRA.*
- Consent to study procedures.
* A repeat baseline MRI/MRA can be performed as a research scan (paid for by study) within 24 hours of enrollment if needed to meet this requirement.
Exclusion criteria:
(listed in the FOCAS protocol) aim to exclude children with contraindications to corticosteroids and children predisposed to stroke of another etiology. Cases of intracranial dissection (FCA-dissection subtype, or “FCAd”) will be included because it is on a spectrum with the inflammatory subtype of FCA (“FCAi”).
Why include children with lenticulostriate infarcts and normal MRA? Lenticulostriate infarct location increases the odds of FCA >40-fold and is present in 76% of FCA cases. It is also seen in 46% of cardioembolic cases, but most of those will be excluded based on risk factors for cardioembolism. (Wintermark M, AJNR 2017).
Intervention:
All children will receive standard therapy of aspirin (5 mg/kg/day, maximum 325 mg/day) and supportive care (to be detailed in FOCAS clinical guidelines). All children randomized to Study Arm A will receive corticosteroids as soon as possible within 24 hours of randomization. Children randomized to Study Arm B will be followed for clinical or imaging evidence of disease progression; the subset with progression will be treated with corticosteroids.
Corticosteroid treatment: standard steroid “pulse” followed by oral taper
- Methylprednisolone IV: 30 mg/kg/day (maximum of 1 g/day) x 3 consecutive days
- Prednisolone po x 4 weeks: 1 mg/kg/d (max 40 mg) x 2 wks, then 0.5 mg/kg/d (max 20 mg) x 2 wks
Determination of FCA Disease Progression:
- All patients will have an MRI/MRA at 5 days post-stroke (±2 days); if no clinical scan is planned, this will be a research scan, paid for by the study
- Additional clinically obtained imaging may also be used to determine disease progression.
- Disease progression will be defined as:
- Radiographic definition of disease progression: worsening FCA severity, defined as an increase in FCASS ≥2 from baseline, with or without clinical changes
- Clinical definition of disease progression: recurrent arterial ischemic stroke (AIS), defined as (a) an increase in pediatric NIHSS ≥2 due either to worsening of prior neurological deficits or new neurological deficits, AND (b) new arterial infarct on brain imaging in a location consistent with the clinical change. A new brain infarct presenting with new focal seizures will also meet criteria for recurrent AIS.
- Because this is a pragmatic comparative effectiveness trial, the local team of treating physicians and clinical radiologists will determine whether a patient meets criteria for disease progression and then initiate corticosteroids as soon as possible (within 12 hours) of that determination. The central FOCAS team can consult as needed.
Outcomes:
- Primary: Change in FCASS (FCA Severity Score) from baseline to 1 month measured on MRAs performed on scanners with the same magnet strength
(e.g., 1.5T versus 3T). - Secondary:
- FCASS at 1 month (required) and 12 months (optional)
- Infarct volume at 1 month (required) and 12 months (optional)
- PSOM (pediatric stroke outcome measure) and “Recovery, Recurrence Questionnaire” (RRQ) at 6 months (required)
Anticipated Enrollment Rate: 3-4 cases per site enrolled over 5.5 years (average of 0.7 cases/site/year)
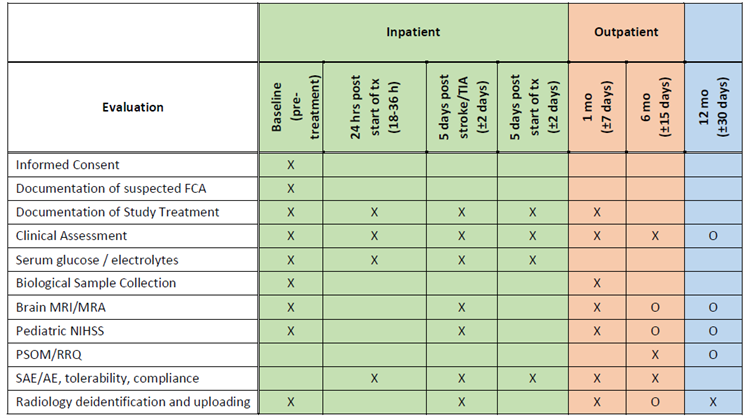
Schedule of Evaluations: The study evaluations will be the same for patients enrolled in both Study Arms.
|
 |
X=required; O=optional (if done/feasible on a clinical basis)
The 5(± 2) day MRI/MRA scan must be performed at least 24-hours following the baseline scan.
The 5(± 2) day post-treatment assessment must be in-person unless the participant has been discharged.
The 6-month clinical assessment can be performed remotely (video/telephone). |